

Water is made up of 2 hydrogens and 1 oxygen.Ģ. The molar mass is calculated by taking the sum of the atomic weights of all the atoms which form the molecule.įirst determine the number of each atom in the formula.ġ. When there is parenthesis's around a group of atoms we must multiply each atom by the subscript to get the total number of each element.Ĭa(NO 3) 2 1 Calcium 2 Nitrogen and 6 Oxygen -> 3 x 2
#OXYGEN MOLAR MASS PLUS#
So the molar mass of glucose is going to be six times the molar mass of carbon plus 12 times the molar mass of hydrogen plus six times the molar mass of oxygen. And so now we have all the information we need from our periodic table of elements. Reading Compounds that have (parenthesis's). Oxygen, we can see from our periodic table of elements, it has a molar mass of 16.00 grams per mole. If there is only 1 atom we do not use subscripts. The subscript belongs to the element it follows and indicates how many of that atom there are.
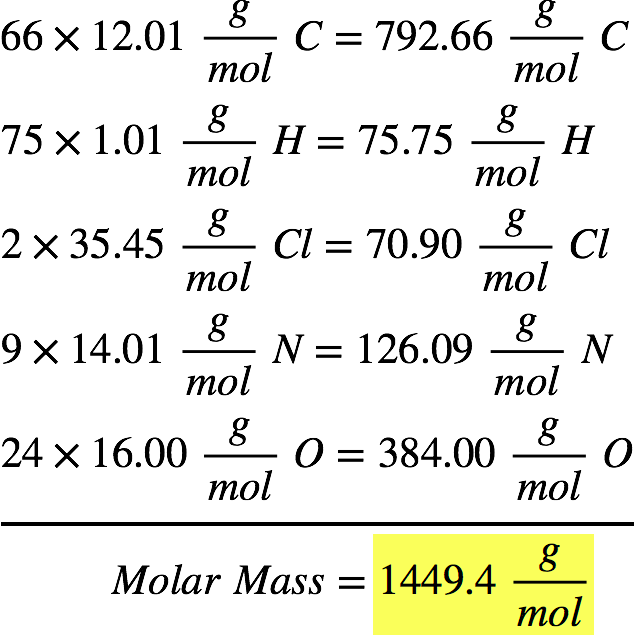
Reading Simple Chemical Formulas- Each element begins with a CAPITAL letter. The units are atomic mass units ( amu ).ġamu =1.660 538 782×10 –27 kg 1 amu is 1/12 the mass of a carbon-12 atom which has a mass of 12.0000. Molecular Mass is the mass of a given molecule (NOT MOLES of molecules). Molecular Weight is the molar mass of a COVALENT compound. The unit for molar mass (note it is the mass of a mole) is grams/mole.Ītomic Weight is the molar mass of an element.įormula Weight is the molar mass of an IONIC compound. Molar Mass is the mass of one mole of a substance (6.02 x 10 23 formula units). (Molar Mass, Molecular Weight, Gram Formula Mass)


 0 kommentar(er)
0 kommentar(er)
